Polycystic kidney disease (PKD) is an inherited disorder strongly linked to mutations in genes PKD1 and PKD2 that causes development of fluid-filled cysts in kidneys of affected patients. According to the National Kidney Foundation, about 600,000 people in the United States have PKD, and it’s the fourth leading cause of kidney failure.
Despite the efforts of many research groups worldwide, there are no cures for PKD, a goal that is hampered by an incomplete understanding of the roots of the disease. Existing animal models have yielded some insight into PKD, but the underlying molecular mechanisms that lead to formation of cysts remain unclear.
Recent research conducted at the University of Pittsburgh School of Medicine and Children’s Hospital of Pittsburgh of UPMC takes aim at this knowledge gap and may help in developing an effective therapy.
Led by Dr. Carlton Bates, chief of the Division of Pediatric Nephrology at Children’s Hospital and professor of pediatrics at Pitt’s School of Medicine, researchers have developed a new genetically modified mouse model that may contribute significantly to understanding PKD.
To create the mouse model, researchers selectively deleted a protein called fibroblast growth factor receptor substrate 2-alpha (Frs2α), which transmits signals for fibroblast growth factor receptors, in a subset of kidney precursor cells in mice. Frs2α, a protein that Dr. Bates has studied extensively, is known to be important for the normal development and patterning of nephrons, which are responsible for blood and waste filtration in kidneys.
Dr. Pawan Puri, a research instructor in Dr. Bates’ lab and the lead author of the study recently published in the journal Scientific Reports, said they were intrigued by the initial observation that mice without Frs2α in nephrons developed kidney cysts early after birth.
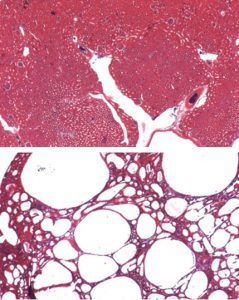
“Looking at kidneys from Frs2α-deficient mice under the microscope we saw that early after birth, the pups developed small cysts that seemed to grow in size and number as the animals matured,” Puri said, added that the observation led researchers to speculate that Frs2α serves as a control switch that prevents kidney cyst formation and allows kidney to develop normally.
Not only did the mouse model show tissue defects similar to that found in PKD, they also displayed abnormalities in their kidney function. The results validated the idea that Frs2α was important to both form and function in kidneys, Puri said.
Researchers also found that Frs2α-deficient mice had high amounts of blood markers known to be increased in acute kidney injury.
“The kidney has an amazing ability to repair following any injury or insult,” Bates said. “Accordingly, our findings suggest that cyst development seen in PKD might be a result of a defective repair process that fails to shut off following kidney injury.”
The researchers believe a new mouse model will greatly benefit the field of kidney research and help develop effective therapies against PKD. Bates noted that his group plans to use the mouse model to further investigate the development of PKD and also study other disease conditions that could be affected by deficiencies in fibroblast growth factor receptors and their signaling proteins such as Frs2a.
Anastasia Gorelova is a Ph.D. candidate in the Department of Pharmacology and Chemical Biology at Pitt’s School of Medicine