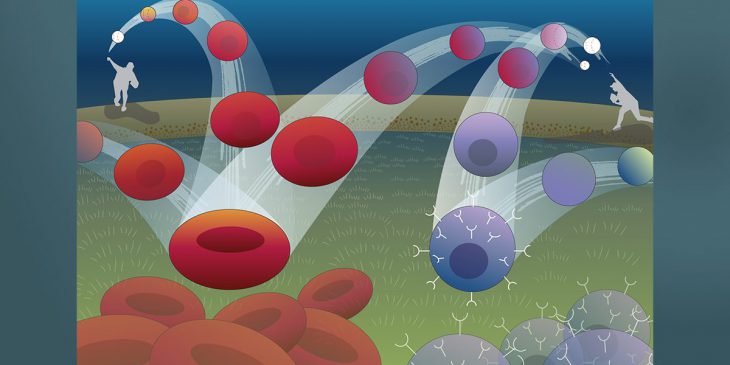
Imagine a ball thrown in the air: It curves up, then down, tracing an arc to a point on the ground some distance away. The path of the ball can be described with a set of simple mathematical equations, and if you know the equations, you can figure out where the ball is going to land.
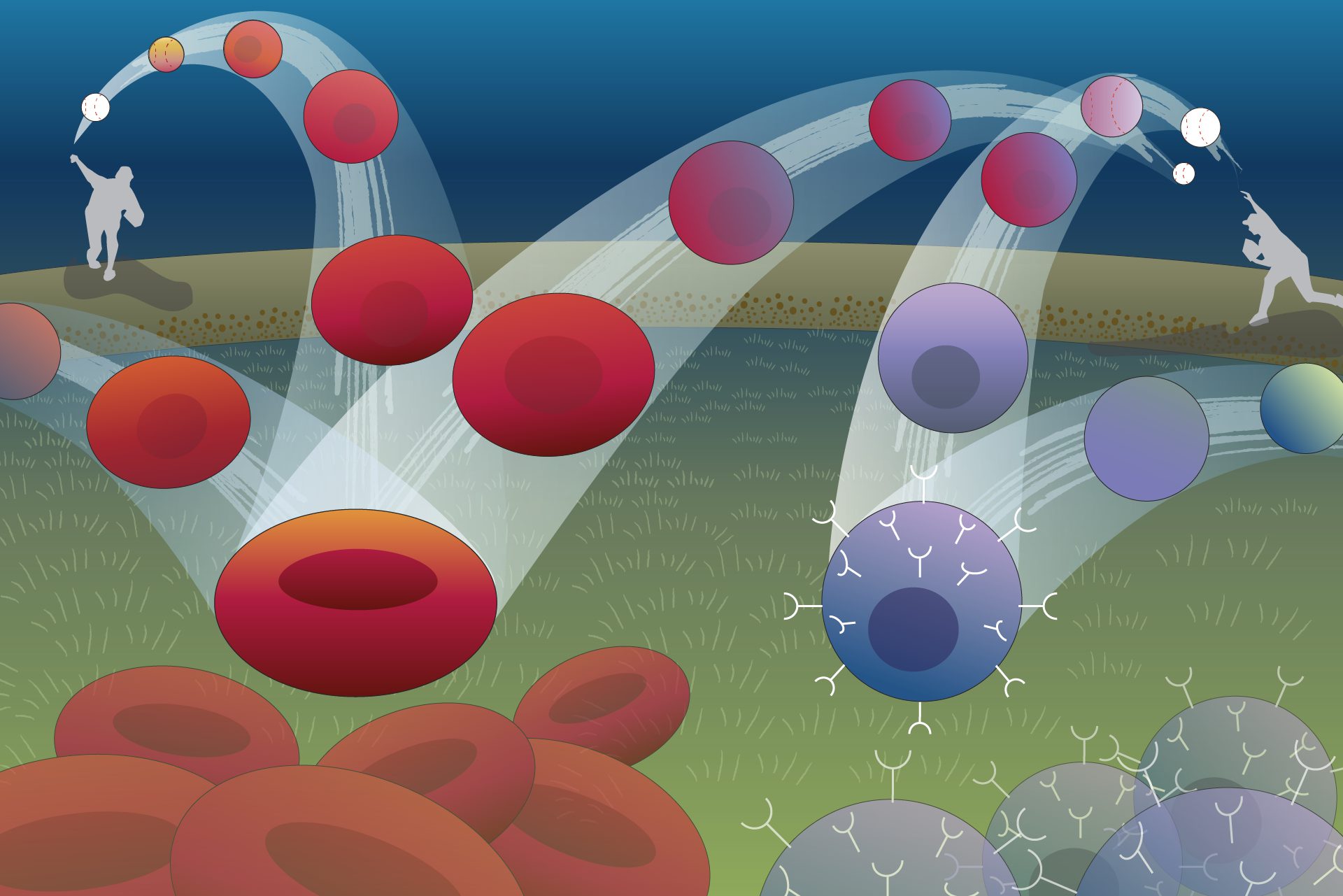
A new computational model makes the path taken by cells as predictable as the arc of a ball
Biological systems tend to be harder to forecast, but researchers from the University of Pittsburgh School of Medicine and collaborators at Whitehead Institute for Biomedical Research are working on making the path taken by cells as predictable as the arc of a ball. Rather than looking at how cells move through space, they are considering how the state of a cell, defined by the expression levels of its genes, evolves with time.

Jianhua Xing, Ph.D.
In a new paper published today in the journal Cell, Dr. Jianhua Xing, professor of computational and systems biology at Pitt, and collaborators describe a computational framework – named Dynamo — that allows them to predict a cell’s fate without performing laborious and costly experiments at the lab bench.
“A cell is a dynamical system that receives and processes a lot of information from the surrounding environment and responds accordingly through a complex regulatory network of genes,” said Xing, whose team includes Dr. Ivet Bahar, professor of computational and systems biology at Pitt, and student Yan Zhang. “If we devise a set of equations that can describe how genes within a cell regulate each other, we can computationally predict how to transform terminally differentiated cells into stem cells or how a cancer cell may respond to various combinations of drugs that would be impractical to test experimentally.”
Along with their collaborators Dr. Jonathan Weissman and Xiaojie Qiu at Whitehead Institute, the Pitt team has developed a machine=learning framework that can construct the mathematical equations describing a cell’s trajectory from one state to another.
The framework also can be used to figure out the underlying mechanisms—the specific cocktail of gene activity—driving changes in the cell. And the researchers hope that Dynamo will not only help them understand how cells transition from state to state, but also guide them in controlling this process.
Dynamo includes tools to simulate how a cell’s gene expression behaviors change based on different manipulations, and a method to find the most efficient way to reprogram any cell type to another — a fundamental challenge in stem cell biology and regenerative medicine — as well as help generate hypotheses of how genetic changes will collectively alter a cell’s fate.
“Our goal is to move towards a more quantitative version of single cell biology,” said Qiu. “We want to be able to map how a cell changes in relation to the interplay of regulatory genes as accurately as an astronomer can chart a planet’s movement in relation to gravity, and then we want to understand and be able to control those changes.”