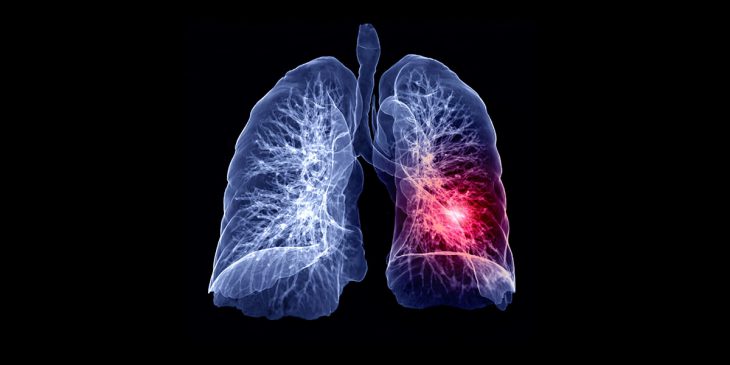
Researchers at UPMC and the University of Pittsburgh School of Medicine are using groundbreaking tumor-highlighting technology to better visualize lung cancer tissue—giving surgeons a significantly better chance of finding and removing more cancer cells than previously possible.
The Enabling LUng Cancer IDentification Using folATE Receptor Targeting (ELUCIDATE) study uses new technology, called OTL38, for patients with suspected lung cancer who are scheduled to undergo endoscopic or thoracic surgery.
OTL38 can detect cancerous tissues not previously identified during preoperative scans. Patients are infused with OTL38 two or three hours ahead of surgery. Fluorescent near-infrared dye and a targeting molecule then attach to receptors on cancer cells and glow under a special camera— guiding surgeons to the precise location of tumor tissue during surgery. This allows for better detection of cancer cells that may not have been visible to the naked eye and better assures the removal of all cancer cells.
“Near-infrared imaging with OTL38 during surgery for lung cancer has the potential to significantly improve the completeness and quality of the operation, therefore improving patient outcomes,” said Dr. Inderpal (Netu) S. Sarkaria, vice chairman for clinical affairs, Department of Cardiothoracic Surgery at UPMC. “It is unique and clinically useful since it is specific to imaging adenocarcinomas, or cancer that starts in the mucous glands of the lung, which is one of the most common types of invasive lung cancer.”
Typically, to determine the size and location of tumors, surgeons use X-rays, MRI, CT scans, PET scans and ultrasound before surgery—but these imaging modalities are rarely available or used during surgery. The ability to see the cancerous tissue in real time may reduce cancer relapse and the need for additional surgeries, as well as increase patients’ overall chances of survival.
Additional study locations include Beth Israel Deaconess Medical Center, Cleveland Clinic, The University of Pennsylvania, MD Anderson Cancer Center, Leiden University Medical Center and On Target Laboratories, LLC.
Click here for further information on the ELUCIDATE Phase 3 trial.